For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Introduction to Enzymes
Effects of Inhibitors on Enzyme Activity
Enzyme inhibitors are substances which alter the catalytic action of the enzyme and consequently slow down, or in some cases, stop catalysis. There are three common types of enzyme inhibition - competitive, non-competitive and substrate inhibition.
Most theories concerning inhibition mechanisms are based on the existence of the enzyme-substrate complex ES. As mentioned earlier, the existence of temporary ES structures has been verified in the laboratory.
Competitive inhibition occurs when the substrate and a substance resembling the substrate are both added to the enzyme. A theory called the "lock-key theory" of enzyme catalysts can be used to explain why inhibition occurs.
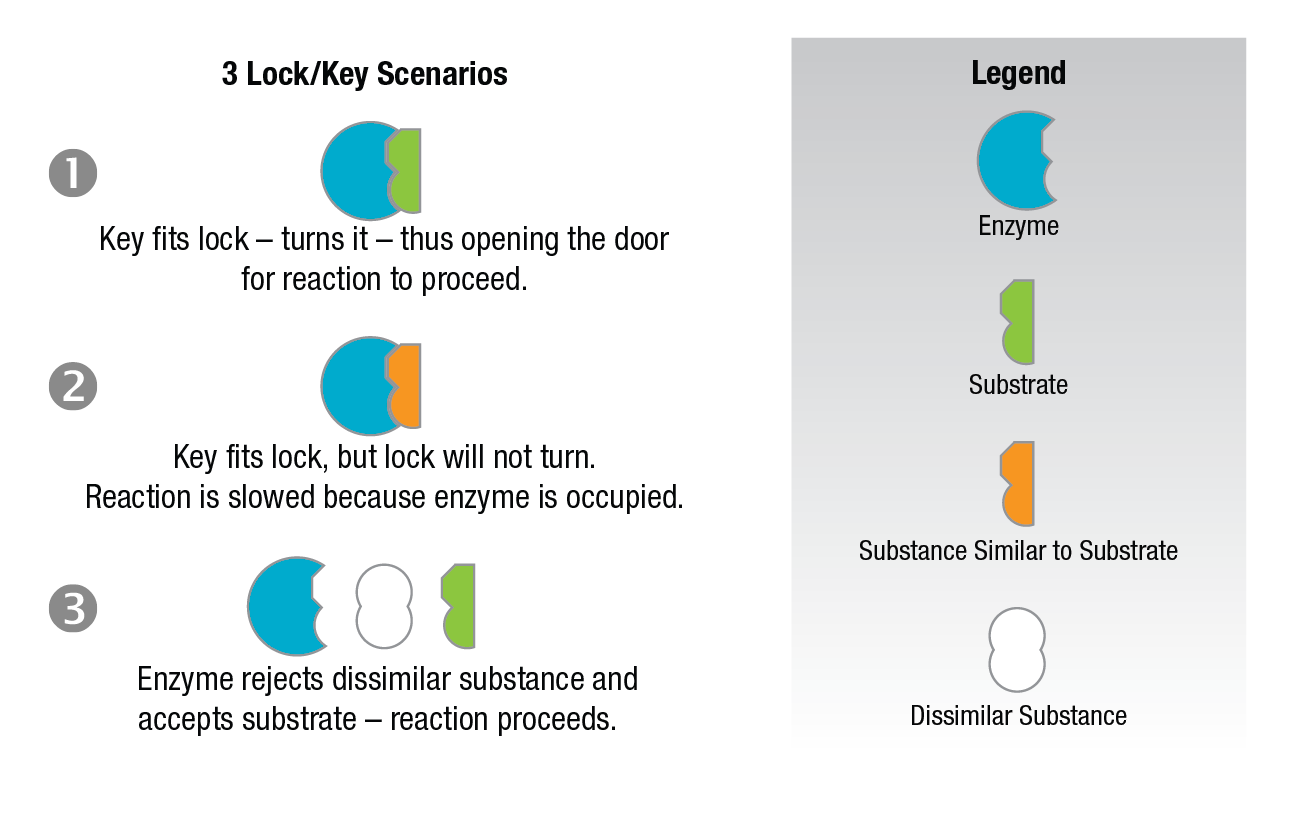
Figure 9: Lock and key theory – competitive analysis.
The lock and key theory utilizes the concept of an "active site." The concept holds that one particular portion of the enzyme surface has a strong affinity for the substrate. The substrate is held in such a way that its conversion to the reaction products is more favorable. If we consider the enzyme as the lock and the substrate the key (Figure 9) - the key is inserted in the lock, is turned, and the door is opened and the reaction proceeds. However, when an inhibitor which resembles the substrate is present, it will compete with the substrate for the position in the enzyme lock. When the inhibitor wins, it gains the lock position but is unable to open the lock. Hence, the observed reaction is slowed down because some of the available enzyme sites are occupied by the inhibitor. If a dissimilar substance which does not fit the site is present, the enzyme rejects it, accepts the substrate, and the reaction proceeds normally.
Non-competitive inhibitors are considered to be substances which when added to the enzyme alter the enzyme in a way that it cannot accept the substrate. Figure 10.
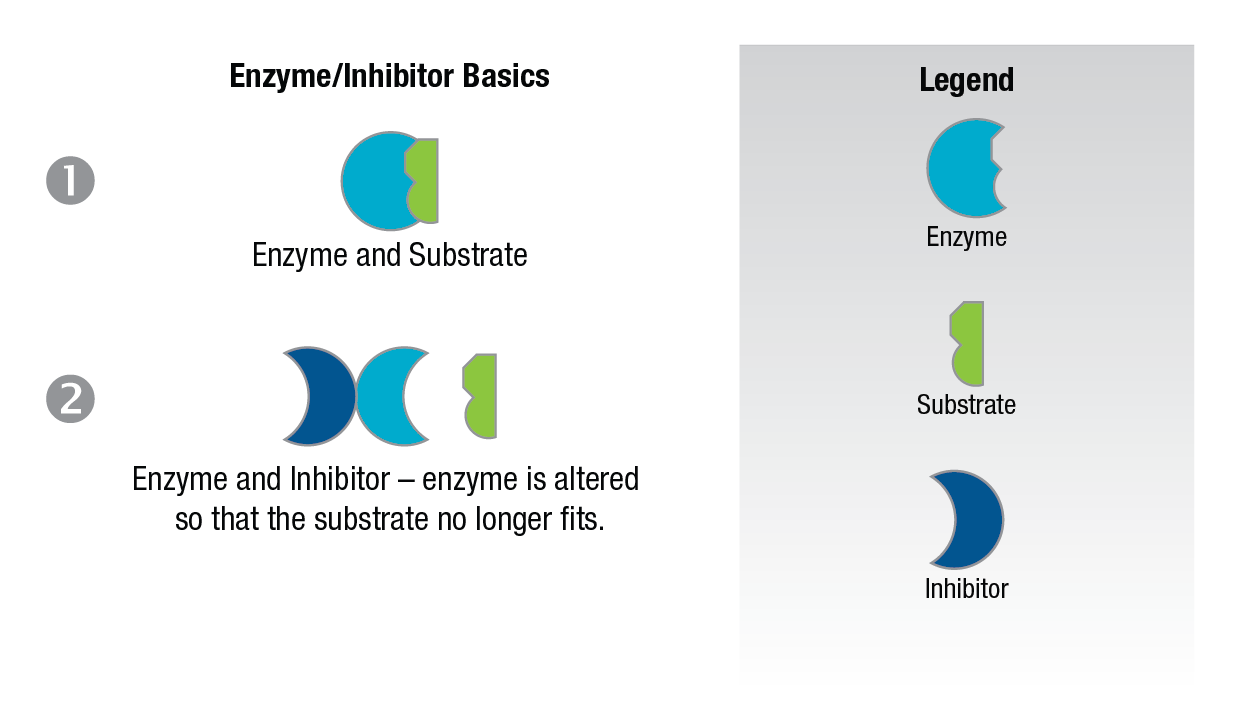
Figure 10: Noncompetitive inhibition.
Substrate inhibition will sometimes occur when excessive amounts of substrate are present. Figure 11 shows the reaction velocity decreasing after the maximum velocity has been reached.
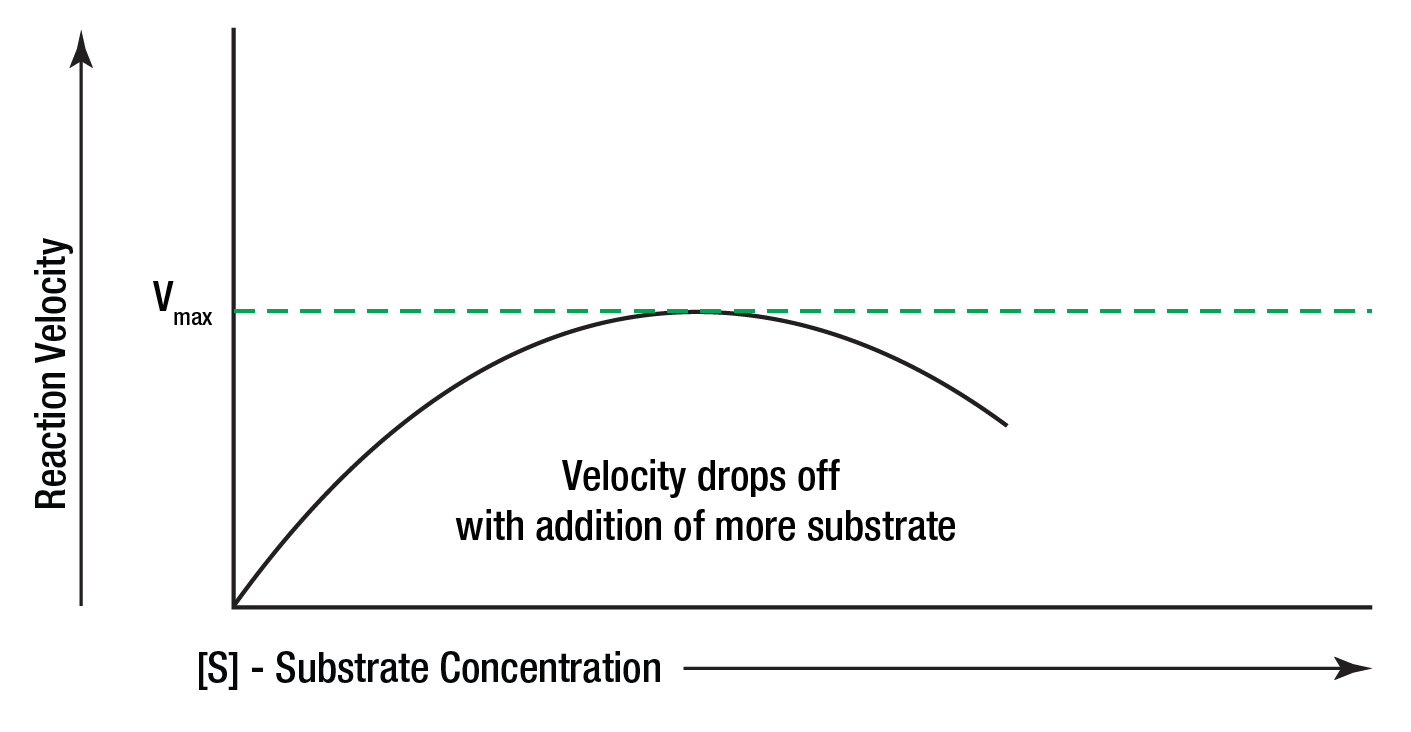
Figure 11: Substrate becoming rateinhibiting.
Additional amounts of substrate added to the reaction mixture after this point actually decrease the reaction rate. This is thought to be due to the fact that there are so many substrate molecules competing for the active sites on the enzyme surfaces that they block the sites (Figure 12) and prevent any other substrate molecules from occupying them.
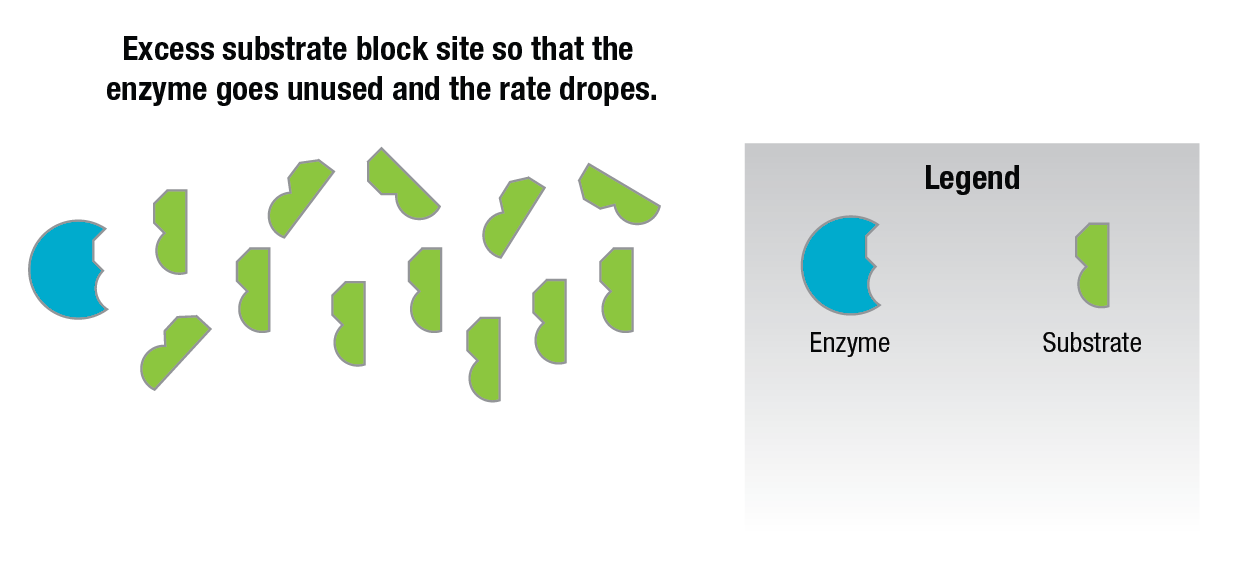
Figure 12: Substrate inhibition.
This causes the reaction rate to drop since all of the enzyme present is not being used.