For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Introduction to Enzymes
Chemical Equilibrium
The study of a large number of chemical reactions reveals that most do not go to true completion. This is likewise true of enzymatically-catalyzed reactions. This is due to the reversibility of most reactions. In general:
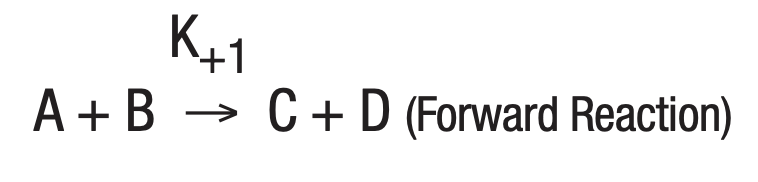 [4]
[4]
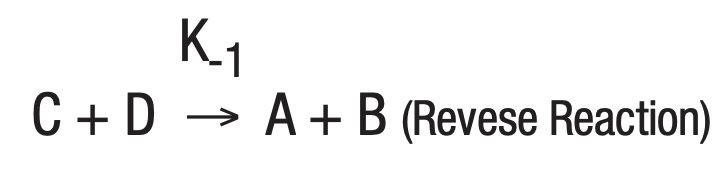 [5]
[5]
where K+1 is the forward reaction rate constant and K-1 is the rate constant for the reverse reaction.
Combining the two reactions gives:
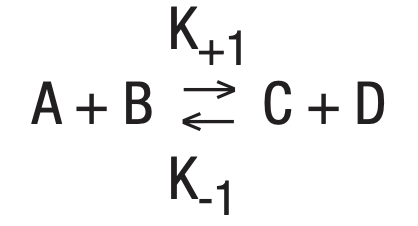 [6]
[6]
Applying this general relationship to enzymatic reactions allows the equation:
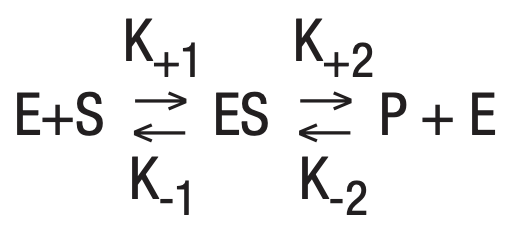 [7]
[7]
Equilbrium, a steady state condition, is reached when the forward reaction rates equal the backward rates. This is the basic equation upon which most enzyme activity studies are based.
File
Introduction to Enzymes
(272.27 KB)