For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Phospholipase A2 - Manual
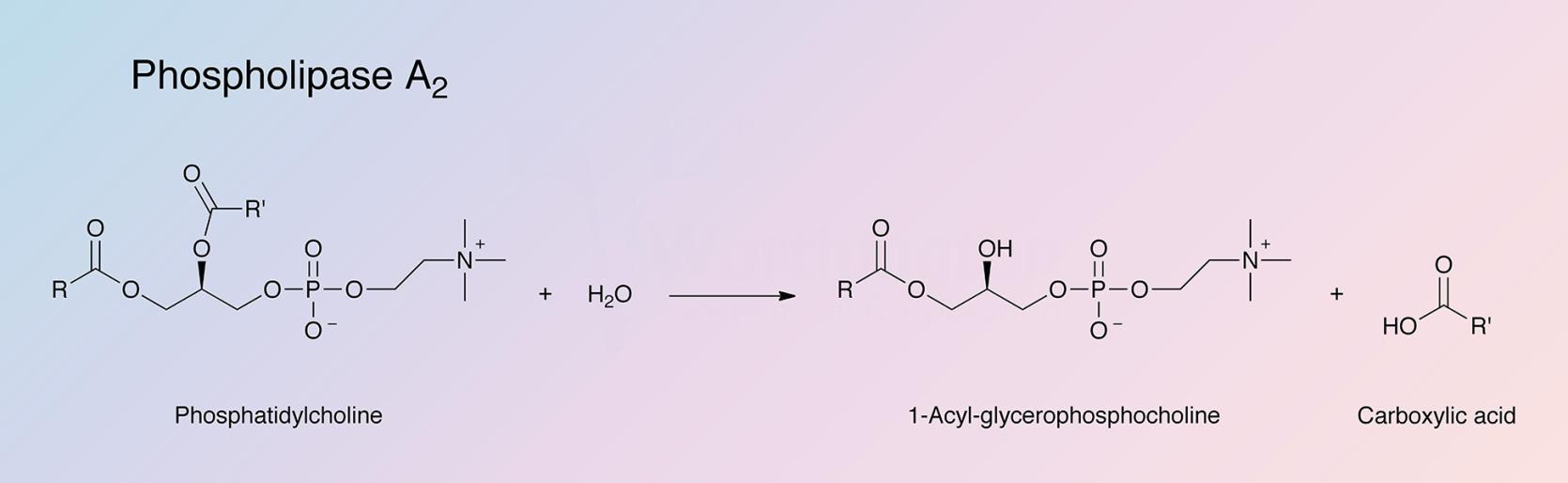
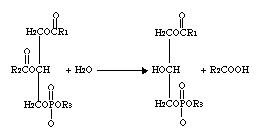
Phospholipase A2 represents a class of heat-stable, calcium-dependent enzymes catalyzing the hydrolysis of the 2-acyl bond of 3-n-phosphoglycerides. This enzyme is named Phospholipase A2 to denote its 2-acyl specificity (Uthe 1971).
Phospholipase A2 has been isolated from pancreas, snake and bee venoms. Forst et al. (1986), Chan et al. (1982), Coulard et al. (1987) and Horigome et al. (1987) report on platelet phospholipase A2. The primary structure of human pancreatic PLA2 has been reported by Verheij et al. (1983).
Phospholipases are involved in lipid metabolism and are important probes of structure-function relationships in biological membranes. Dawson 1973).
The enzyme specifically catalyzes the hydrolysis of the β-fatty ester linkage of L-α phosphoglycerides (Van Deenen 1963).
Two proteins (A2-α, A2-β) exhibiting Phospholipase A activity have been isolated from Crotalus adamanteus (Saito and Hanahan 1962, and Wells and Hanahan 1969). Each protein is composed of dimeric subunits (Wells 1971). The two proteins have similar activities but are chromatographically and electrophoretically distinct. Heinrikson et al. (1977) report on the distinctive structural features of the two enzymes and the complete amino acid sequence of phospholipase A2-α. The two subunits differ at a single amino acid; A2-α contains glutamine at residue 117, while the more acidic A2-β contains glutamic acid at this residue.
P00623
Molecular weight: 30,000 (Wells 1969)
Extinction coefficient: ![]() = 22.7 (Wells 1969)
= 22.7 (Wells 1969)
4.55 and 4.40 for A-α and A-β, respectively (Saito 1962)
Calcium ion (Dennis 1973)
Zinc, barium, and manganese ions (Golec et al. 1992, and Uthe 1971)