For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Phosphatase, Acid - Manual
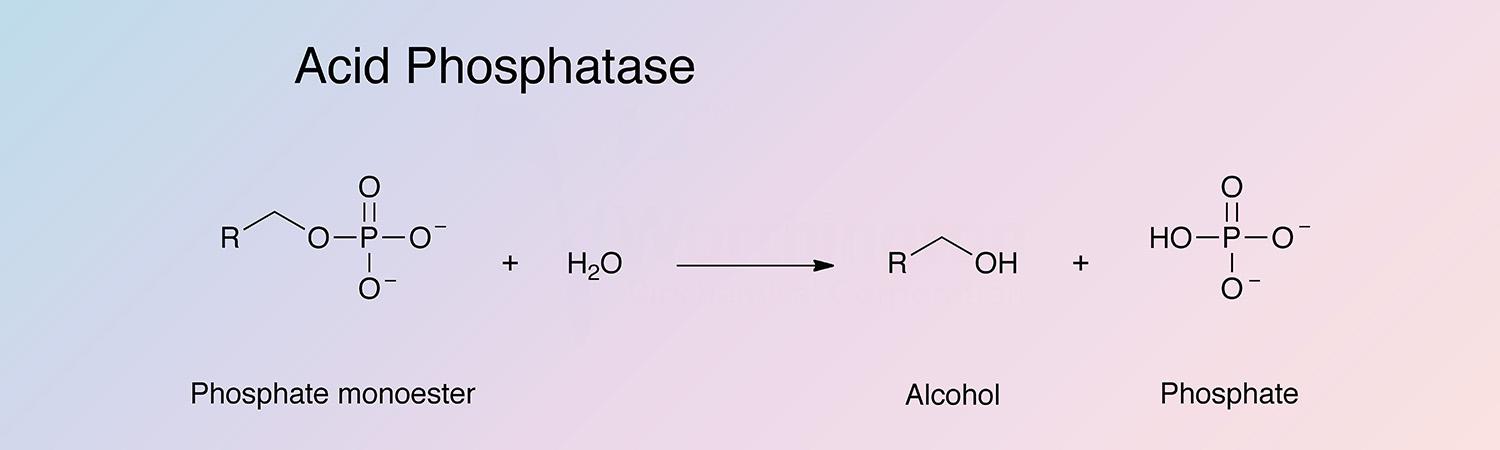
Orthophosphoric-monoester phosphohydrolase (acid optimum)
Acid phosphatase catalyzes the following reaction at an optimal pH below 7:
Acid phosphatase is ubiquitous in nature, perhaps one of the most concentrated sources being human prostate gland, a fact exploited by the clinical chemist who measures the serum enzyme level as an index of prostatic cancer. It has also been associated with Gaucher's disease, patients exhibiting unique peaks in their electrophoresed sera (Goldberg et al. 1966). Acid phosphatases have been reviewed by Hollander (1971) although the wheat germ enzyme is not included.
Acid phosphatase activity was observed by Teller in 1954 in preparations of a wheat germ lipase described by Singer in 1948. Subsequent work confirmed that the non-specific esterase activity of the wheat germ preparation may be measured both as lipase (tracetin as substrate) and phosphatase.
The enzyme has been purified and its properties described by at least three different laboratories. Joyce and Grisolia (1960) purified it some 3000-fold and measured its activity on a variety of phosphate esters. Brouillard and Ouellet (1965) obtained four separate forms of the enzyme on ion-exchange and gave evidence for the presence of ferric iron in the molecule. Verjee (1969) obtained three active peaks and showed them to have individual properties.
Characteristics of Acid Phosphatase from Wheat Germ:
The enzyme has a broad esterase activity. See Joyce and Grisolia (1960). It shows highest activity for pyrophosphate.
Three isozymes of closely similar molecular weights have been reported by Verjee (1969): EI, EII, and EIII. See also Brouillard and Ouellet (1965).
55,000 ± 5,000 (Verjee 1969).
EI - 5.5, EII - 4.5, and EIII - 4.0. (Verjee 1969).
Fluoride, molybdate and orthophosphate (Verjee 1969).