For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Neuraminidase - Manual
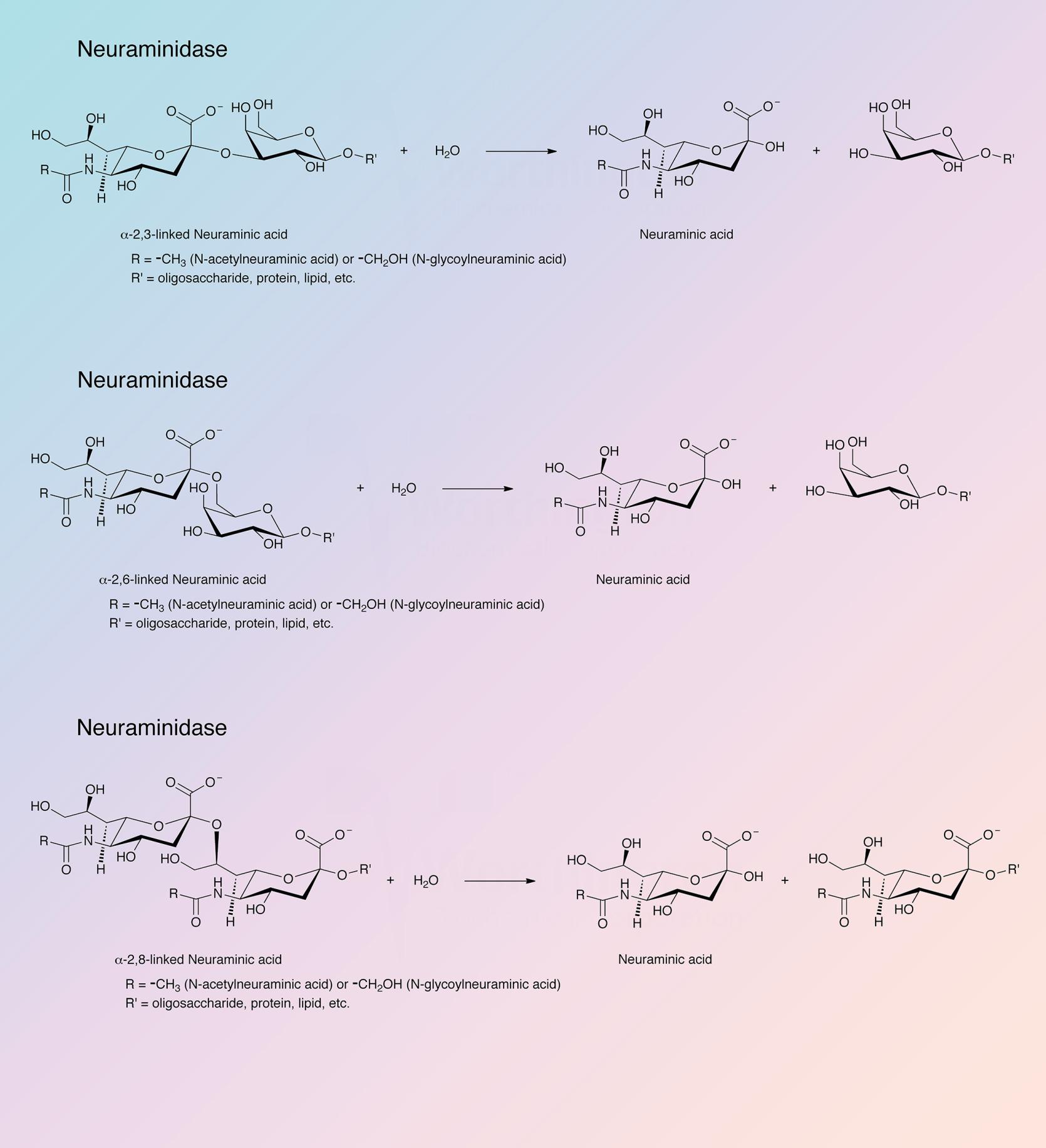
Neuraminidase (sialidase) splits off N-acetyl neuraminic acid (sialic acid) from a variety of glycoproteins.
![]()
Neuraminidases are important tools for the study of glycoproteins in protein chemistry and cell biology (Hatton et al. 1973).
Worthington neuraminidase is derived from Clostridium perfringens. Chromatographic purification has been developed in this laboratory based on work of Cassidy et al. (1965). Wooley and Gommi (1966) describe the use of this neuraminidase in a method for measuring serotonin receptors. It is also employed to determine bound N-acetylneuraminic acid in tissues.
Characteristics of Neuraminidase from Clostridium perfringens:
5.0-5.1 (Burton 1963). Little or no activity at pH 4.0 or above pH 8.0.
5.1 (Groome and Belyaven 1958).
None, in contrast to the neuraminidase from Vibrio which has a divalent metal requirement.
Considerable interest has been shown in using neuraminidase inhibitors as possible anti-viral and anti-bacterial agents. (Khorlin et al. 1970; Haskell et al. 1970; and Tute 1970).
The purified preparation is stable for at least two years when stored at 4°C (Cassidy et al. 1965).
Serum albumin, 0.3 mg/ml (Cassidy et al. 1965).