For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Amino Acid Oxidase, L- - Manual
L-amino acid oxidase (LAAO) catalyzes the oxidative deamination of a number of L-amino acids, predominantly hydrophobic and aromatic L-amino acids. LAAO represents approximately 30% of the total venom of some snake species (Takatsuka et al. 2001).
LAAO was first discovered by Zeller and Maritz (Zeller and Maritz 1944, 1945). It was found to occur in almost all snake venoms and designated a flavoprotein in 1948 (Zeller 1948).
LAAO was first prepared in crystalline form in 1958 by Wellner and Meister. In 1960, Wellner and Meister studied properties of the enzyme including prosthetic groups, electrophoretic fractions, stability, and pH dependence. In this study, they also isolated LAAO of Agkistrodon piscivorus piscivorus to compare it to that of C. adamanteus. Soon after, the enzyme mechanism was studied and further details of the mechanism were elucidated (Wellner and Meister 1961, and Massey and Curti 1967). The kinetics of the oxidase reaction were also investigated (Radd 1964, and Zeller et al. 1965).
In the 1970s vinylglycine was determined to be a suicide substrate/inactivator of LAAO (Marcotte and Walsh 1976), and the effect of pH and competitive inhibitors was also studied (de Kok and Veeger 1968). LAAO of C. adamanteus was the first LAAO found to act as a bactericidal agent, a property that is still being studied today (Skarnes 1970, and Zuliani et al. 2009). The gene sequence was determined in 1998 by Raibekas and Massey.
Recent work has involved using LAAOs to study apoptotic and cytotoxic effects (Stábeli et al. 2007), and LAAO is also being studied for its use as an anti-parasitic agent (Sant’Ana et al. 2008).
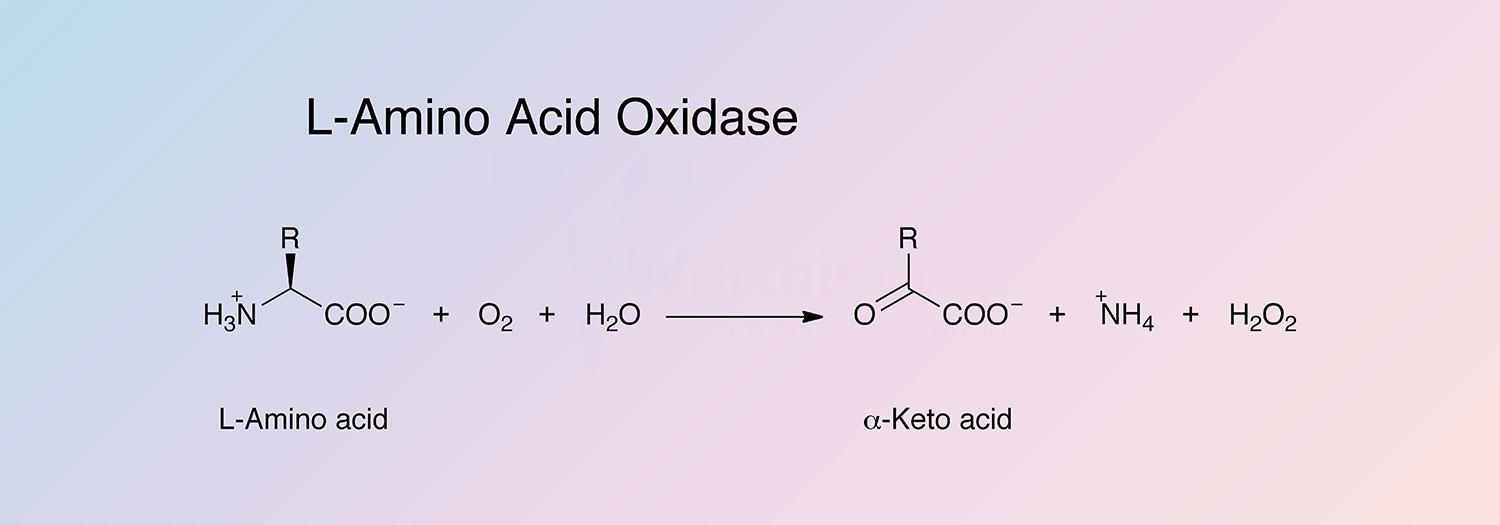
LAAO is specific for L-isomers. Substrates are the L-isomers of leucine, isoleucine, norleucine, alpha-amino butyric acid, phenylalanine, tyrosine, tryptophan norvaline, methionine, histidine, and citrulline. Histidine and tyrosine cannot be determined in an L,D-mixture (Malmstadt and Hadjiioannov 1963). Methylene blue may be used as an electron acceptor. L-serine, threonine, aspartic acid, glutaric acid, lysine, and ornithine are deaminated only to a limited extent.
LAAO is believed to contain three hydrophobic substrate binding sites, designated a, b, and c. Subsite a accommodates one methylene carbon, b two, and c three. An amino binding subsite is designated d (Tan et al. 1991). This model is used to explain why amino acids with branching at the second carbon are unable to accommodate into subsite a, and are oxidized slowly or not at all (Zuliani et al. 2009).
LAAOs are FAD-dependent enzymes and are usually homodimeric, binding glycoproteins with molecular masses of 110-150 kDA. Important residues identified in C. rhodostoma LAAO include Glu63, Arg71, and Glu457, which interact with the FAD molecule. The dimethylbenzene ring cofactor is surrounded by Ile374, Trp420, and Ile430; Lys326 coordinates a water molecule (Pawelek et al. 2000). These residues have been found to be conserved in the majority of snake venom LAAOs (Pawelek et al. 2000, and França et al. 2007).
LAAOs are widely distributed, being found in bacteria, fungi, green algae, plants, and snake venom. LAAOs of snake venom show a high degree of sequence homology, with conservation of at least 13 of the 24 N-terminal amino acids that are believed to be involved in substrate binding. (Zuliani et al. 2009). The N-terminal sequence contains a highly conserved beta-alpha-beta-fold domain involved in FAD binding (Du et al. 2002).
- Purification and determination of certain amino acids (Nicholson and Kim 1975)
- Preparation of alpha-keto acid (Nicholson and Kim 1975)
- Assaying peptidase activity (Nicholson and Kim 1975, and Donlon and Fottrell 1971)
- Oxidation reactions
- Catalyst in supercritical CO2 (Findrik et al. 2005)
O93364
LAAO has three domains:
- Class: Alpha Beta; Alpha Beta; Mainly Alpha
- Architecture: Alpha-Beta Complex; 3-Layer(bba) Sandwich; Orthogonal Bundle
- Topology: Polyamine Oxidase (Chain A, domain 2); FAD/NAD(P)-binding domain; Guanine Nucleotide Dissociation Inhibitor (domain 1)
113.3 kDa (Theoretical)
Approximately 7.5 for leucine (Wellner and Meister 1960)
6.32 (Theoretical)
- 124,900 cm-1 M-1 (Theoretical)
- E1%, 275 = 17.9
- Cyanide
- Ammonium and benzoic acid (Meister and Wellner 1963)
- Aromatic carboxylates (de Kok and Veegar 1968)
- Benzoic acid derivatives (de Kok and Veeger 1968)