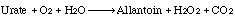For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Uricase - Manual
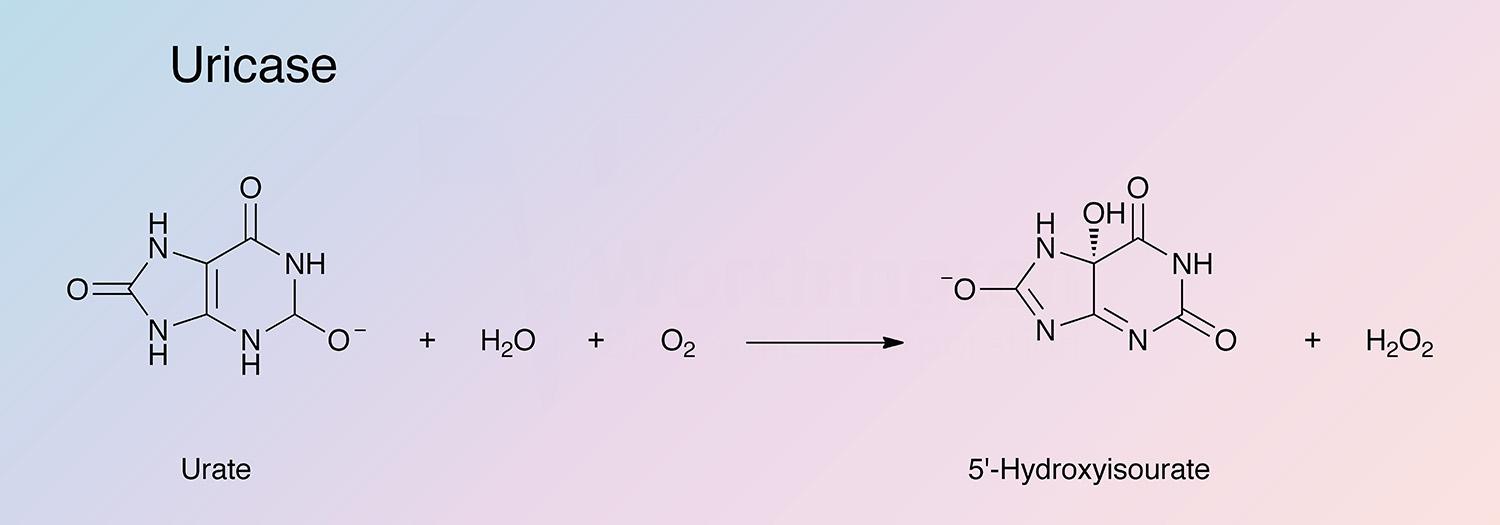
Uricase (urate oxidase) catalyzes the following overall reaction:
This reaction represents the termination of purine catabolism in all mammals excluding man, higher apes and the Dalmatian dog.
The enzyme has been reviewed by Mahler (1963).
Uricase is very important for the determination of uric acid in biological fluids. Not only is the reaction specific, it may be monitored at 292 nm, 340 nm or colorimetrically by coupled chromogenic response. Non-enzymatic methods are interfered with by turbidity or the presence of aspirin, ascorbic acid, glutathione, paracetanol and many antibiotics. See Itiaba et al. (1975); Kuan et al. (1975); Pesce et al. (1974); Gökicke and Gökicke (1973); Kabasakalian et al. (1973); Lum and Gambino (1973); Steele (1970) and Troy and Purdy (1970).
Characteristics of Uricase from Hog Liver:
The enzyme is highly specific for uric acid. (See Mahler 1963).
The enzyme is composed of four subunits of 32,000 M.W. There is one copper atom per molecule (125,000 M.W.) (Pitts et al. 1974).
125,000 (Pitts et al. 1974).
9.0 (Mahler 1963).
6.3 (Mahler 1963).
= 11.3 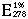 (Mahler 1963).
(Mahler 1963).
Various purine analogues of urate (Bergmann et al. 1963; Baum et al. 1956), cyanide and other copper chelating agents. Fridovich (1965) reports that urate in alkaline solution may be converted to a potent inhibitor, oxonate.
Purified uricase in 10% saturated ammonium sulfate is stable for a year at 5°C.