For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Peroxidase - Manual
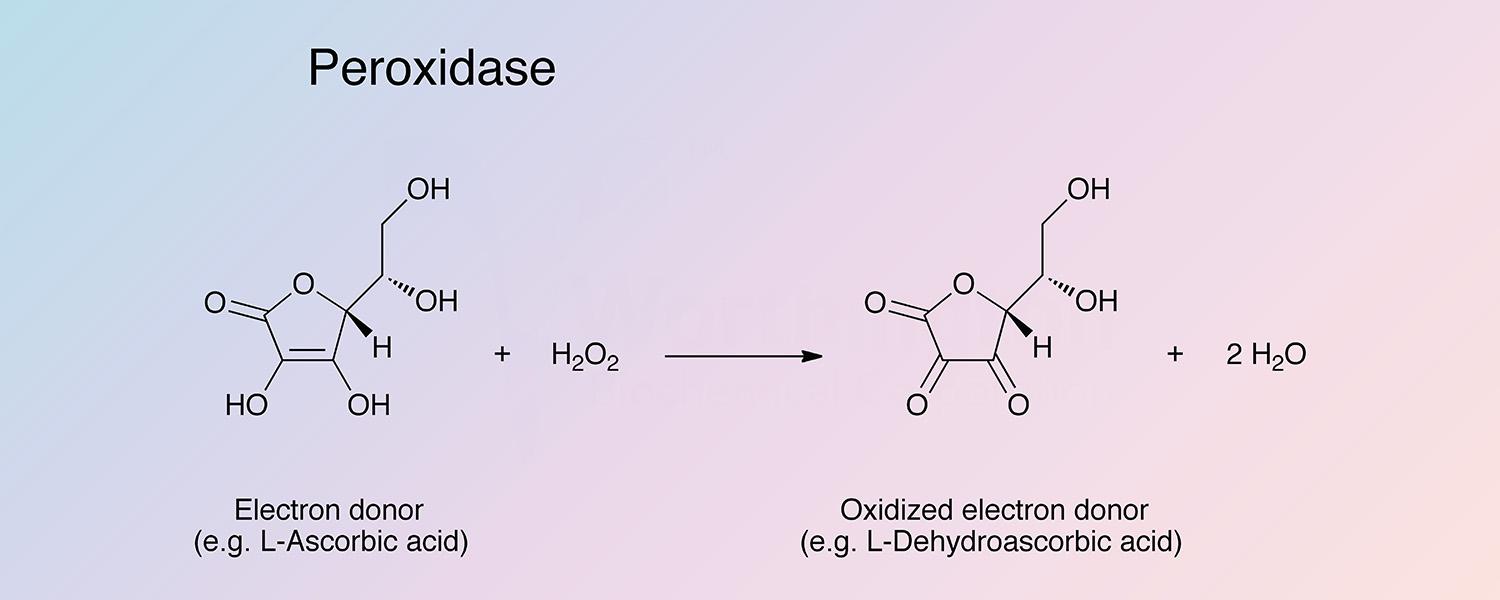
Peroxidase (HRP) is a hemoprotein catalyzing the oxidation by hydrogen peroxide of a number of substrates such as ascorbate, ferrocyanide, cytochrome C and the leuco form of many dyes.
Peroxidase has been found well suited for the preparation of enzyme conjugated antibodies, due in part to its ability to yield chromogenic products, and in part to its relatively good stability characteristics. Peroxidase labeled immunoglobulins have been used successfully as immunohistological probes for the demonstration of tissue antigens, and in enzyme amplified immunoassay systems for the quantitative determination of soluble and insoluble antigens (Nakane and Pierce 1967; Avrameas 1969; Kurstak et al. 1969; Avrameas and Guilbert 1972; van Weeman and Schuurs 1974; Greenwalt et al. 1975). Sternberger et al. (1970) and Moriarty et al. (1973) have described novel soluble peroxidase-antiperoxidase techniques for immunohistochemistry and immunoassay.
The use of the highly specific, sensitive and very stable horseradish peroxidase with a chromogenic donor has proven very useful for assay systems producing hydrogen peroxide; for example, in the determination of glucose or galactose by their respective oxidases and in the determination of certain L-amino acids in conjunction with L-amino acid oxidase (Malmnstadt and Hadjiioannou 1963). A more generalized application of the principle is as follows:
To a solution of 0.05 M sodium acetate, pH 5.0, add 10 mg o-dianisidine hydrochloride, 18.6 mg EDTA, 5.0 ml of a filtered 0.1% peroxidase solution (equivalent to Worthington Code: HPOD), and 1.0 ml 10% Triton X-100. Dilute to 50 ml with sodium acetate. Protect solution from light. Add 2.0 ml of reagent to 2.0 ml of unknown. After 15 minutes at room temperature, read absorbance at 460 nm. Standard solutions containing from 1 to 3 micrograms of hydrogen peroxide per test are run simultaneously. Alternatively, the extinction coefficient of oxidized o-dianisidine may be used. It has been determined to be 1.13 X 104 M-1 cm-1.
A fluorometric method of determining H2O2 in concentrations as low as 5 X 10-12 mole/ml has been described by Perschke and Broda (1961).
Characteristics of Peroxidase from Horseradish Roots:
The enzyme exhibits a high specificity. Activity is observed with H2O2, MeOOH, and EtOOH (Maehly and Chance 1954). See also Chmielnicka et al. (1971) and Morrison and Bayse (1973).
Seven isozymes have been described by Shannon et al. (1966);Kay et al. (1967); and Strickland et al. (1968). See also Delincée and Radola (1975) and Shih et al. (1971). All contain photohemin IX as prosthetic group. Neutral and amino sugars account for approximately 18% of the enzyme. Weinryb (1966) indicates that the "active site" involves apoprotein as well as the heme group. See also Lanir and Schejter (1975). Dolman et al. (1975) have reported on the formation of Compound I. See also Dunford et al. (1975), Santimone (1975) and Stillman et al. (1975).
40,000 (Maehly 1955).
7.0 (Maehly 1955).
7.2 (Maehly 1955).
Horseradish peroxidase is reversibly inhibited by cyanide and sulfide at a concentration of 10-5 M (Theorell 1951).
The enzyme is quite stable. As a lyophilized powder, it may be stored several years refrigerated.