For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Hexokinase - Manual
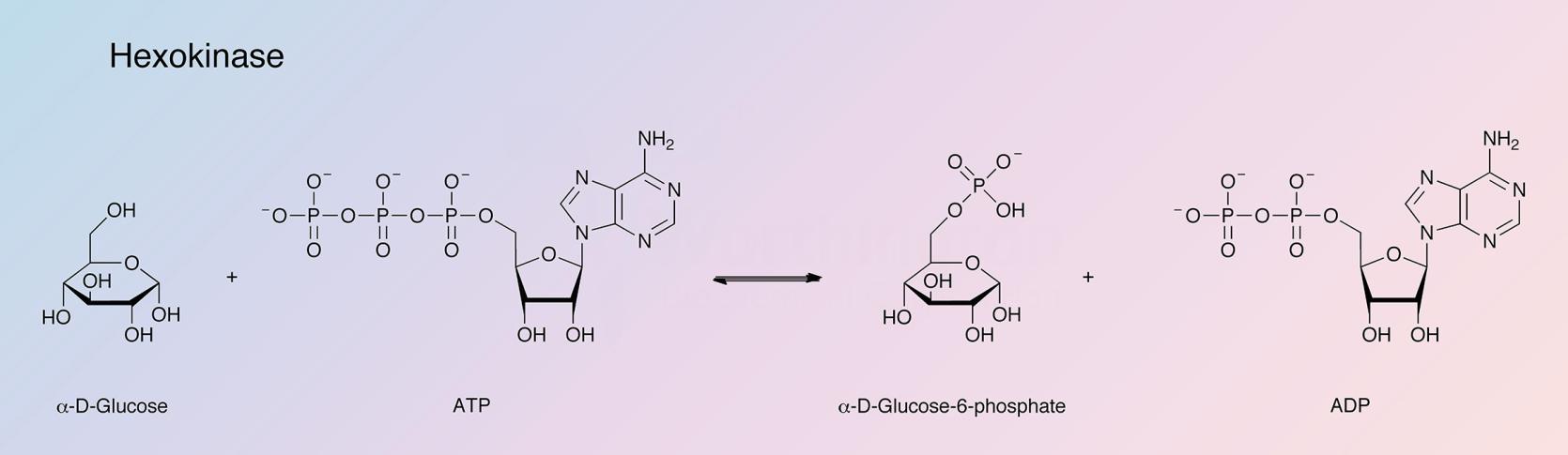
Hexokinase catalyzes the reaction:
Hexokinases have been isolated from the yeast cell in two distinct forms, designated P-I and P-II (Schulze et al. 1969). These are separate, noninterconvertible isozymes (Womack et al. 1973).
Hexokinase is used to determine glucose, fructose, mannose and ATP.
Characteristics of Hexokinase from Yeast:
The enzyme phosphorylates D-fructose, 5-keto-D-fructose (Avigrad et al. 1968), D-glucose, 2-deoxy-D-glucose, D-mannose and D-glucosamine. ATP and ITP have been demonstrated to transphosphorylate in the yeast hexokinase reaction (Martinez 1961). The activity of P-I with fructose is 2.6 times that with glucose, whereas with P-II, a fructose:glucose ratio of 1:3 is obtained (Lazarus et al. 1966). The substrate specificities of yeast hexokinase have been extensively studied by Bessell et al. (1972).
Both P-I and P-II contain the same amino terminus, valine, and the same carboxy terminus, alanine. Amino acid composition has been reported by Schmidt et al. (1973b).
The native forms have molecular weights of about 100,000 (Schulze et al. 1969) and consist of polypeptide chains of molecular weights slightly higher than 50,000 (Schmidt et al. 1973).
7.5 - 9.0 (Sols et al. 1958).
P-I, 5.25 and P-II, 4.93 (Schmidt et al. 1973).
![]() = 8.85 for P-I and 9.47 for P-II (Schmidt et al. 1973).
= 8.85 for P-I and 9.47 for P-II (Schmidt et al. 1973).
Hexokinase requires magnesium ions for its catalytic activity. It is activated by catecholamines and related compounds (Harrison et al. 1972). Calcium ions do not affect the enzymatic activity.
The enzyme is inhibited by compounds which react with SH groups. It is also inhibited by sorbose-1-phosphate, polyphosphates, 6-deoxy-6-fluoroglucose, 2-C-hydroxy-methylglucose, xylose and lyxose (Sols et al. 1958 and McDonald 1955).
Both the lyophilized preparation and the crystalline suspension are stable for 6-12 months at 2-8°C.