For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Glyceraldehyde-3-Phosphate Dehydrogenase - Manual
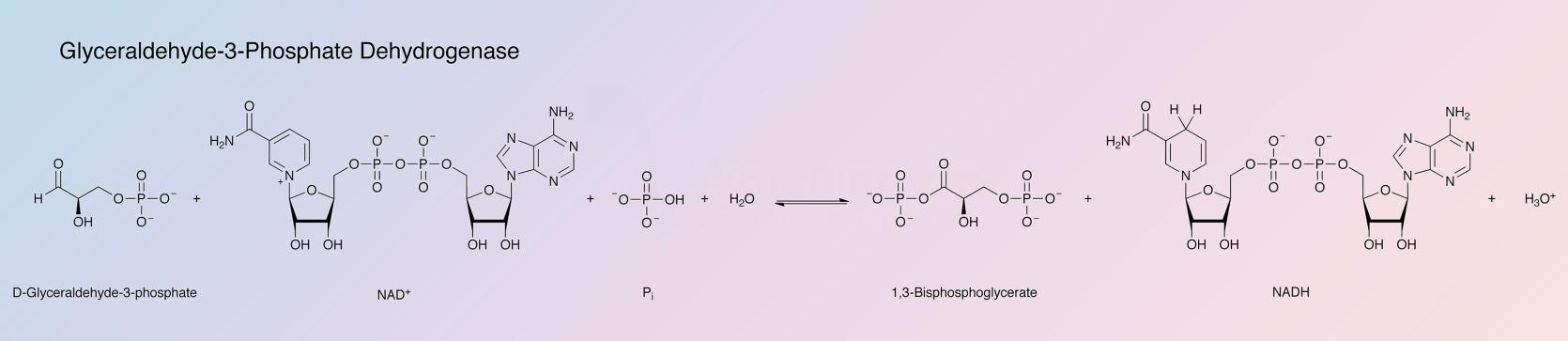
Glyceraldehyde-3-phosphate dehydrogenase (GAPD) catalyzes the following reaction:
See Hill et al. (1975a), Fife and Szabo (1973), and Francis et al. (1973).
GAPD is a key enzyme in intermediary metabolism catalyzing the oxidation and subsequent phosphorylation of substrate aldehydes to acyl phosphate. Buehner et al. (1973) have indicated its structural similarity to LDH, MDH and ADH. It occurs in high concentrations in mammalian, fish, and fowl muscle, yeast and bacteria. The human enzyme has been reported on by Watson et al. (1972) and Girotti (1976); that of pig muscle by Harris and Perham (1965) and Batke et al. (1974); lobster GAPD the amino acid sequence of which is reported to be like that of rabbit, by Davidson et al. (1967). Yeast GAPD amino acid-sequence was determined by Jones and Harris (1972). A report on rabbit muscle GAPD purification is that by Hill et al. (1975b). Nagradova and Grozdova (1974) report on the close structural similarity of the enzymes from mammals. The hybridization of GAPD subunits of different origin forms mixed tetramers, even between such diverse sources as rabbit muscle and Ascaris (Kochman et al. 1974), and rabbit muscle and yeast (Spotorno and Hollaway 1970; Osborne and Hollaway 1974 and 1975). See also Markovich and Krapivinskaya (1975). Suzuki and Harris (1975) report on hybridization of GAPD subunits from rabbit, pig, lobster, yeast and E. coli and indicate that individual subunits retain their activity even within tetramers formed with subunits from other species.
The enzyme is used for the determination of D-Gly-3-P, NAD+, Pi, ATP, glycerate-3-P and glycerate-1,3,diP. It is also used for the determination of phosphoglycerate kinase activity.
Characteristics of GAPD from Rabbit Muscle:
The normal dehydrogenase reaction involves NAD as coenzyme, phosphate and D-glyceraldehyde-3-phosphate as substrates. Arsenate is often used to replace phosphate in assays. D-glyceraldehyde is very slowly oxidized.
The enzyme consists of four identical polypeptide chains (MW 36,000) each accommodating one molecule of NAD+ as coenzyme (Reisler et al. 1975). See also Price and Radda (1974). The monomers are joined noncovalently. Hoagland and Teller (1969) indicate that as a dimer GAPD exhibits esterase activity and that the dimer and tetramer may be in equilibrium. See also Constantinides and Deal (1970).
Allison et al. (1973) report on a sulfenic acid form of GAPD that functions as an acetyl phosphatase. The active site involves cysteine 149 which, in the presence of NAD+ and the substrate aldehyde, forms a thiol ester intermediate (Bode et al. 1975). See also Reisler et al. (1975). Bloch et al. (1971) indicate that GAPD prepared according to Ferdinand (1964) binds ADP-ribose rather than NAD+. See also Hill et al. (1975). Other active site reports include Dwek et al. (1975), Boers and Verhoeven (1973), Buehner et al. (1973) and Levitzki (1973) on the similarity of binding sites to LDH, MDH, and ADH.
144,000 (See Bode et al. 1975).
pH 6.55 (Cori et al. 1948). See also Velick and Furfine (1963).
 = 10.2 (Murdock and Koeppe 1964).
= 10.2 (Murdock and Koeppe 1964).
EDTA, cysteine, and Cleland's reagent have activating or protective effects. NAD binding is considered to have allosteric effects (Kirschner et al. 1966), to cause conformational changes (Havsteen 1965; Listowsky et al. 1965; Jaenicke and Gratzer 1969), to reverse inhibition by multivalent anions (Fenselau 1970), and to decrease susceptibility to proteolysis (Fenselau 1970).
Reagents reacting with or oxidizing the catalytic site sulfhydryl group inhibit the enzyme. Heavy metal ions, pCMB, iodoacetate, o-iodosobenzoate, and tetrathionate are among known inhibitors. ATP, cyclic AMP, and other adenine-containing compounds have been indicated as resulting in reversible dissociation, competitive inhibition, and increased susceptibility to proteolysis (Stancel and Deal 1968, 1969; Constantinides and Deal 1969; Yang and Deal 1969; Fife and Szabo 1973; Oguchi et al. 1973; Hixson and Hixson 1975; Smith et al. 1975).
Crystalline GAPD is stable for one year when refrigerated as a suspension in 2.9 M ammonium sulfate solution.