For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Diaphorase - Manual
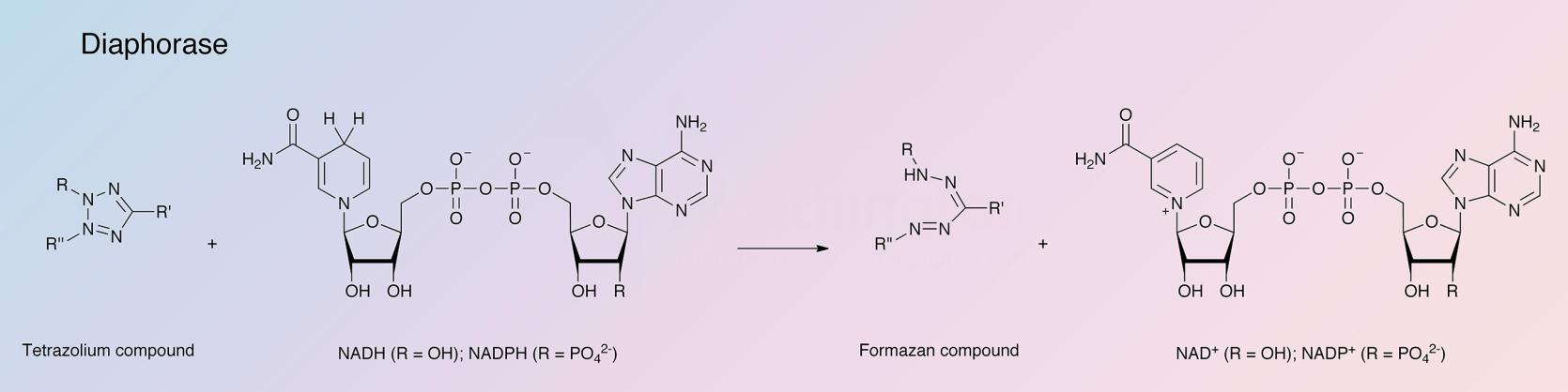
NADPH: (acceptor) oxidoreductase
The diaphorases are a ubiquitous class of flavin-bound enzymes that catalyze the reduction of various dyes which act as hydrogen acceptors from the reduced form of di- and tri- phosphopyridine nucleotides, i.e., NADH, NADPH. The first such enzyme to be purified was that from heart muscle (Straub 1939). Almost twenty years later heart diaphorase was shown to be identical to lipoyl dehydrogenase (Massey 1958, 1963). Other diaphorases have been described and purified from various bacteria, plants and mammalian organs. Diaphorase activity of a partially purified extract of Clostridium kluyveri cells, originally described as a source of NADH and NADPH oxidase (Ciotti and Kaplan 1957), was observed in this laboratory and its use applied to the coupled, colorimetric determinations of dehydrogenases and ethanol. (Teller 1958). These methods, based on the decolorization of 2,6-dichlorophenolindophenol were improved by the substitution of a tetrazolium dye which becomes chromogenic on reduction. (Brower and Woodbridge 1970; Nachlas et al. 1960).
The enzyme from Cl. kluyveri has been purified and characterized by Kaplan, Setlow, and Kaplan (1969), who used starting material equivalent to the present Worthington product.
Characteristics of Diaphorase from Clostridium kluyveri:
Unless otherwise noted, the following data have been obtained from Kaplan et al. (1969).
Either NADH or NADPH may be used as reductants. However, no exchange of hydrogen between the coenzymes is catalyzed. Neither oxygen nor cytochrome C is reduced by Cl. kluyveri diaphorase.
The enzyme contains one molecule of flavin mononucleotide per molecule. The amino acid composition has been determined.
Q97E86 (Clostridium acetobutyliucum)
24,000
8.5. At pH 7.4 and 9.4 the activity is reduced 50%. Both NADH and NADPH share this optimum.
We have found that excess flavin mononucleotide (FMN) appears to stimulate the reaction with dichlorophenol-indophenol slightly.
N-ethylmaleimide inactivates diaphorase at concentrations of under 5 mM.
Though of reasonable stability, if held for prolonged periods, the dry enzyme should be stored in a freezer. Solutions, especially, should always be kept out of strong light.
NADH, NADPH and FMN protect the enzyme against denaturation by urea and guanidine.
Km for NADH was reported to be 9 X 10-5 and for NADPH it was much lower.