For our international customers, please be advised that orders cannot be placed through our website by customers in countries with International Distributor representation.
Cholinesterase, Butyryl - Manual
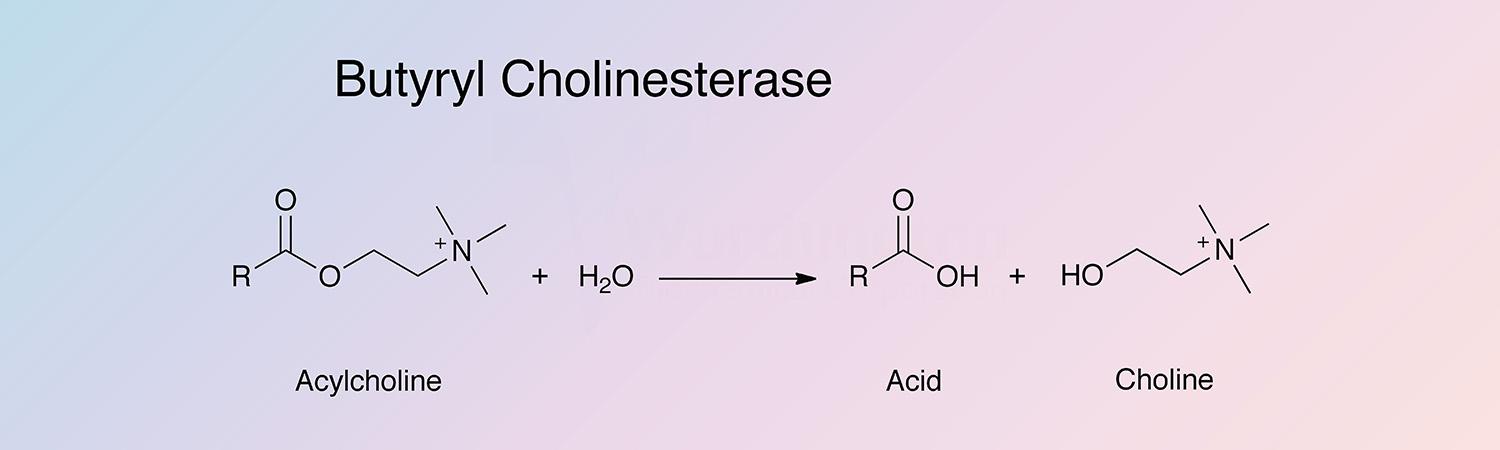
Acylcholine acylhydrolase
Butyryl cholinesterase, (ButChE), catalyzes the hydrolysis of a number of choline esters:
The enzyme is found in mammalian blood plasma, liver, pancreas, intestinal mucosa and the white matter of the central nervous system. It is sometimes referred to as serum cholinesterase as opposed to red cell cholinesterase (AChE). It hydrolyzes butyrylcholine 4 times more rapidly than acetylcholine. ChE does not hydrolyze D-β-methyl acetylcholine whereas AChE does. Both are inhibited by 10-5 M physostigmine. Because the enzyme is so markedly inhibited by organophosphate compounds used as insecticides and neurotoxins, it is widely used in monitoring systems. An immobilized cholinesterase detection device has been described by Goodson et al. (1973).
Characteristics of Butyrylcholinesterase from Horse Serum:
The enzyme is more active with butyryl and propionyl choline than with acetyl choline. See also Main et al. (1974) for some comparative activities. Non-choline esters (e.g., procaine, morphine esters, atropine and cocaine) are susceptible to the action of ButChE (Augustinsson 1960).
Lee and Harpst (1973) indicate ButChE to be a tetrameric structure with equally sized subunits of 110,000 daltons. It is a glycoprotein. Main et al. (1974) have determined the amino acid and carbohydrate composition, based upon a particle weight of 101,000. See also Chiu et al. (1972) and Pavlic (1972).
440,000 (Lee and Harpst 1973; see also Main et al. 1974)
6.0-8.0 (Augustinsson 1960)
E280 =13.6 (Main et al. 1974)
Ca2+ and Mg2+ (Augustinsson 1960)
Numerous organophosphate esters, the carbamate derivatives and quaternary ammonium salts (Augustinsson 1960). See also Kamaric (1975), Koelle et al. (1974), Millner et al. (1974), Stanley et al. (1974), Ashani et al. (1972), Post (1971).